European operations
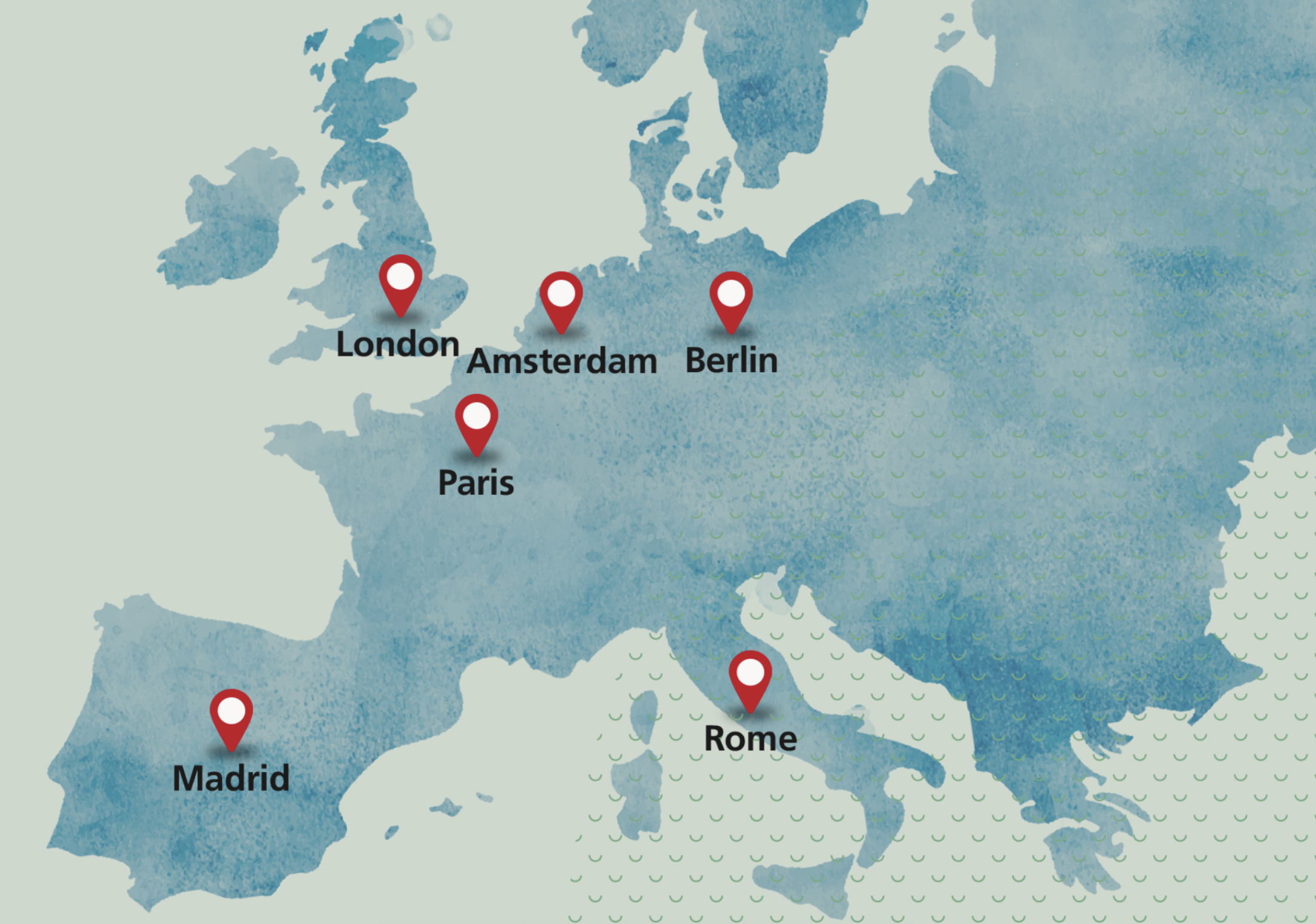
Our regional corporate Headquarters in Amsterdam, opened in November 2022, sits at the centre of our European operations, along with an additional operational hub in London, making the most of the thriving life science communities across both biopharmaceuticals and medical technology in these countries, and of course the presence of key regulatory bodies such as the European Medicines Agency (in the Netherlands).
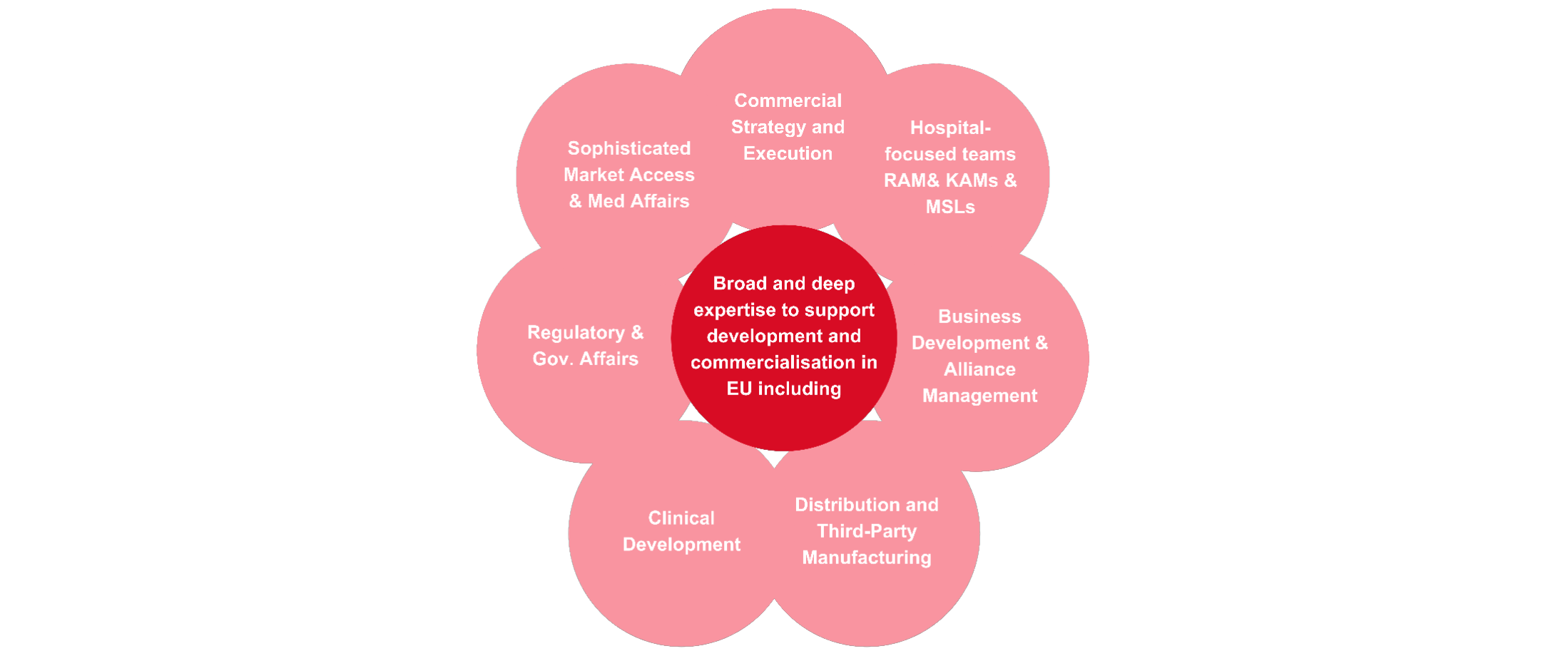
Core capabilities to successfully navigate EU healthcare system
We have significant expertise to enable the development and commercialisation of treatments across Europe. The diagram below lists some of our core capabilities, from clinical development and distribution through to market access and medical affairs